Every discipline in high school has its individual but fundamental principles – rules or laws – that students must learn so they can progress. It’s rarely just ‘one’ thing in a subject but there are constants or “frequent flyers” as a mentor teacher once called them.
In English class, it might be rules of grammar. In math, it’s the reliability and requirement of proof, or maybe it’s a variety of formulae. Bertrand Russell however would remove this simplicity and instead write 59 chapters to explain seven different fundamental principles!
Meanwhile, scientists (and science teachers) could create their own long list. However, for today let us declare that one of those fundamentals is gas laws! Knowing the key scientists from history and their laws that govern the behavior of gases under different conditions is in fact fundamental for student learning.
If you are looking for a COMPLETE PowerPoint and student-guided note-taking sheet for the characteristics and properties of gases, pressure and temperature conversions, and ALL gas laws, this is it! Properties of Gases and Gas Laws PPT & guided notes.
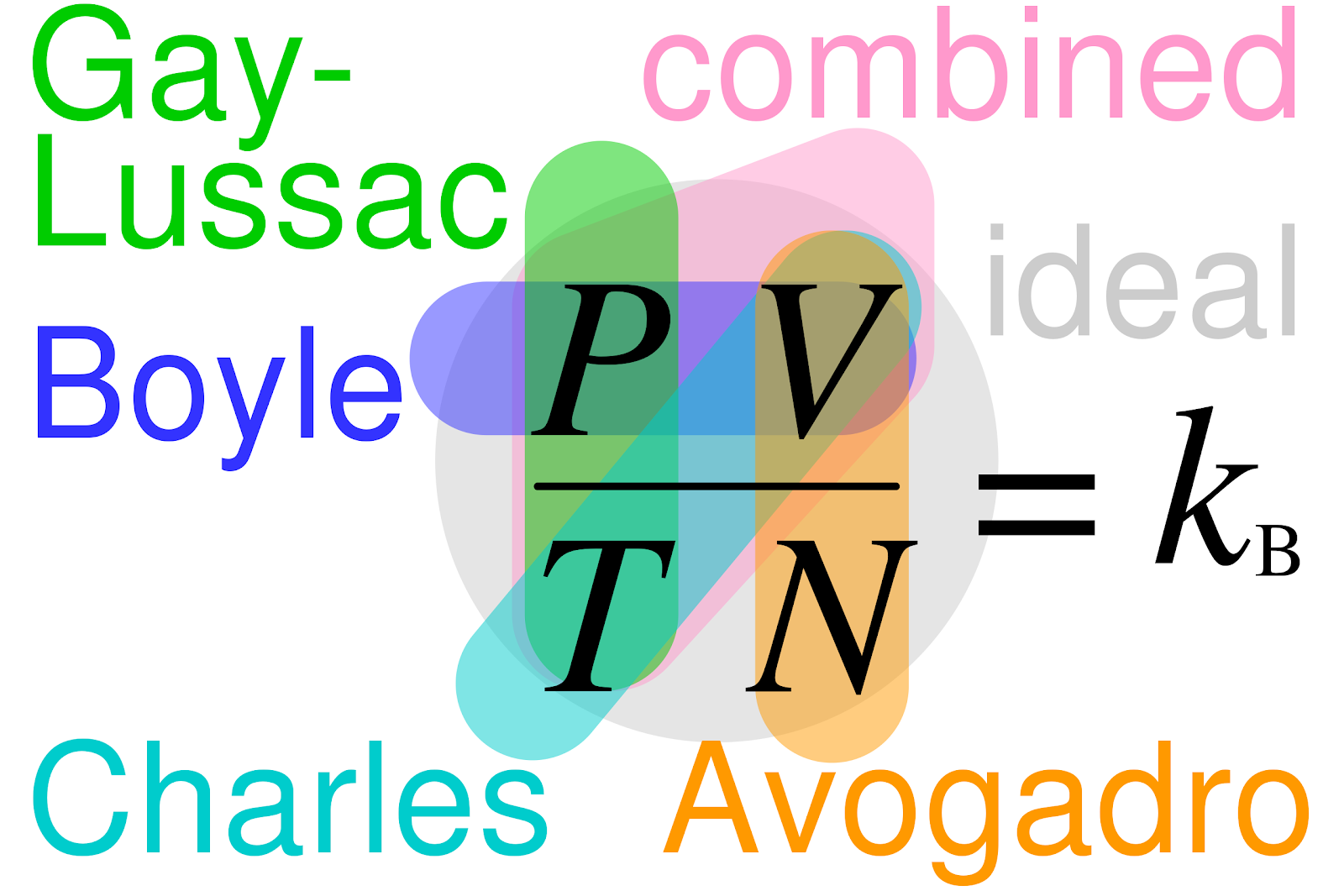
Developed in the early 1800s Avogadro’s Law is named for Italian physicist Amadeo Avogadro. This law explains how gases behave in everyday situations.
Avogadro’s Law is also a key component of the combined gas law.
But this is only one of several gas laws! Check out this post for an overview of all of the gas laws. And for more details on the individual laws and NGSS-aligned teaching approaches check out this post with a deep dive into Boyle’s Law and Charles’s Law and this post with details about Gay-Lussac and the Combine Gas Laws.
This concept remains as relevant now in the 21st century (if not more so) because understanding the application of Avogadro’s Law means scientists can make better predictions to ensure enhancements in real life and technology.
For example, we can better understand air pollution and move closer to finding viable solutions or it can help design cars to ensure safety and better efficiency, thereby helping to improve air pollution/air quality.
For engaging and effective demonstrations and real-world applications to teach students the concepts of Avogadro’s Law and the Ideal Gas Law keep reading!
The Return of the Mole: Avogadro’s Law
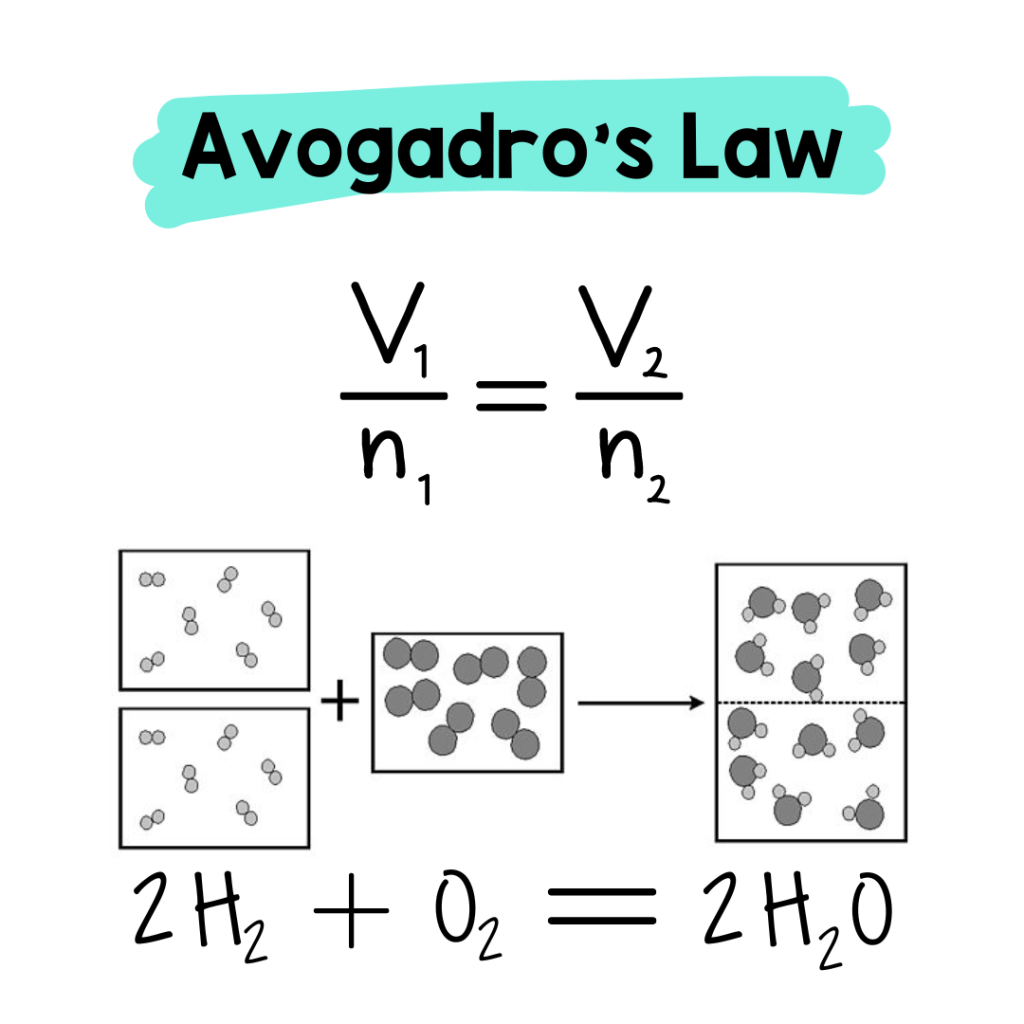
Avogadro’s Law states that a given number of molecules of any substance occupies the same volume at constant pressure and temperature. Increase or decrease the number of molecules, the volume will increase or decrease, too (respectively). It is a direct relationship.
Get your students to practice Avogadro’s Law practice problems with this freebie.
Real-life examples demonstrating Avogadro’s Law
Beyond practice with problems, it’s important that students can connect Avogadro’s Law to real-life examples. One way is to examine Avogadro’s Law and Environmental Science and Meteorology.
Understanding gas volume is relevant to atmospheric science and the study of air quality. Avogadro’s Law, tied to the amount of gas, connects to the composition of the atmosphere. It’s instrumental in understanding how different gases, contributing to air quality, interact and vary in concentration.

Another option is to complete a demonstration using baking soda and vinegar in bottles. Include varying amounts across three bottles and see if students can guess which bottle has the least reactants based on the size of the balloon.
Here’s a great demo of this in action.
These real-life applications are directly tied to NGSS’s Science and Engineering Practices (SEP) and Crosscutting Concepts (CCC). Students can explore the planning and carrying out investigations SEP as well as analyzing and interpreting data. For CCCs, it’s simply a matter of making connections between cause and effect or energy and matter.
Once students understand Avogadro’s Law, it is the missing piece for the final gas law, which is the Ideal Gas Law.
Bringing It All Together (The Ideal Gas Law)
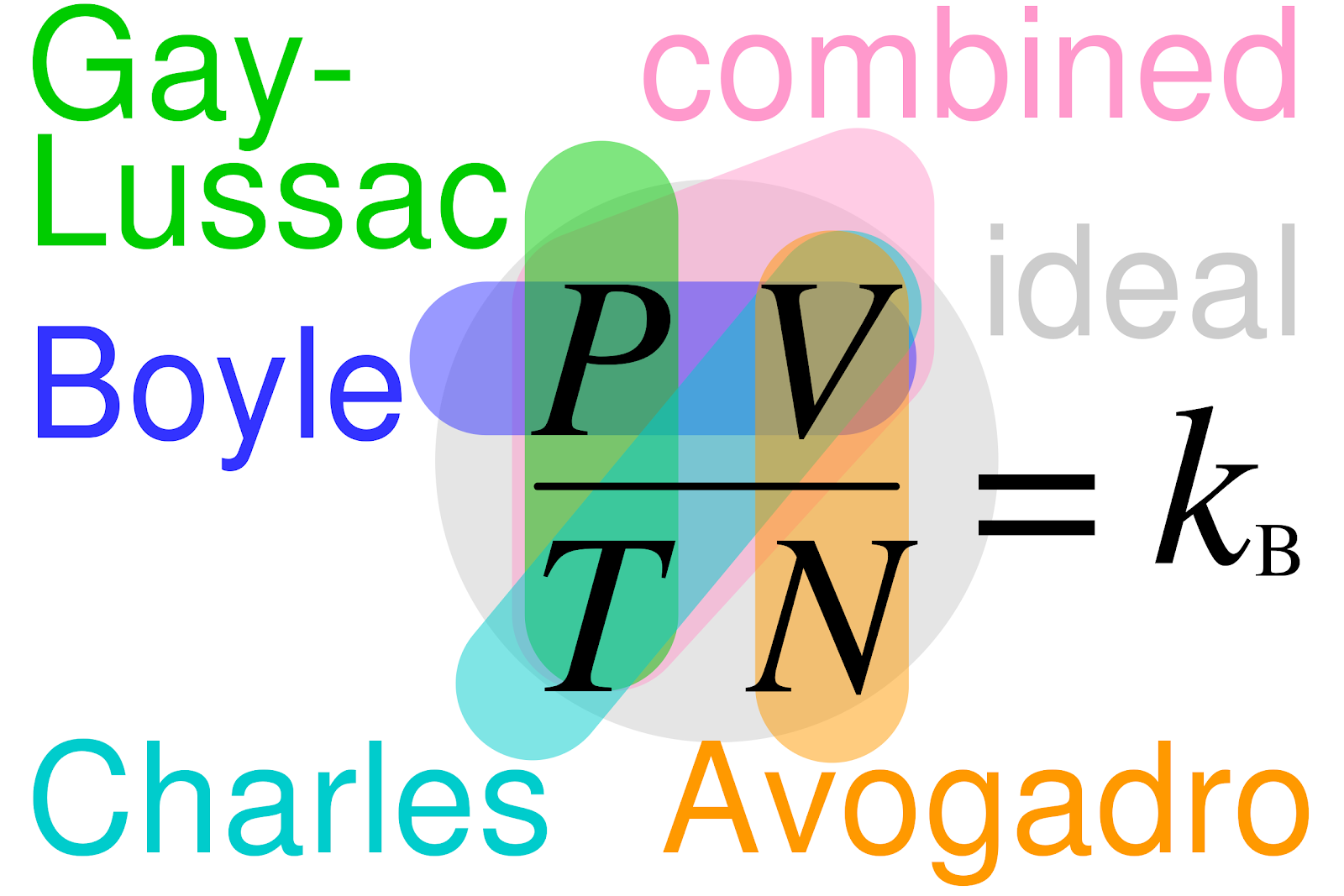
The Ideal Gas Law brings everyone to the party! The combination of the four key laws by Gay-Lussac, Boyle, Charles, and Avogadro creates the Ideal Gas Law declaring that PV = nRT
The ideal gas constant (R) is a fundamental physical constant that appears in the Ideal Gas Law equation. Its value depends on the units of pressure, volume, and temperature. The most common units for the ideal gas constant are:
- 0.0821 L⋅atm/(mol⋅K) (when pressure is in atmospheres, volume in liters, and temperature in Kelvin),
- 8.314 J/(mol⋅K) (when using SI units, with pressure in pascals, volume in cubic meters, and temperature in Kelvin).
The Ideal Gas Law therefore has its own practical applications. Most notable is the takeaway that gases do not behave “ideally” so the Ideal Gas Law gives scientists the ability to predict the behavior of gases.
Investigate this concept with this set of ideal gas law practice problems, which goes over the relationship between pressure, volume, temperature, and moles of a gas. And it includes a set of teacher answers with all work shown, along with how to set up each problem.
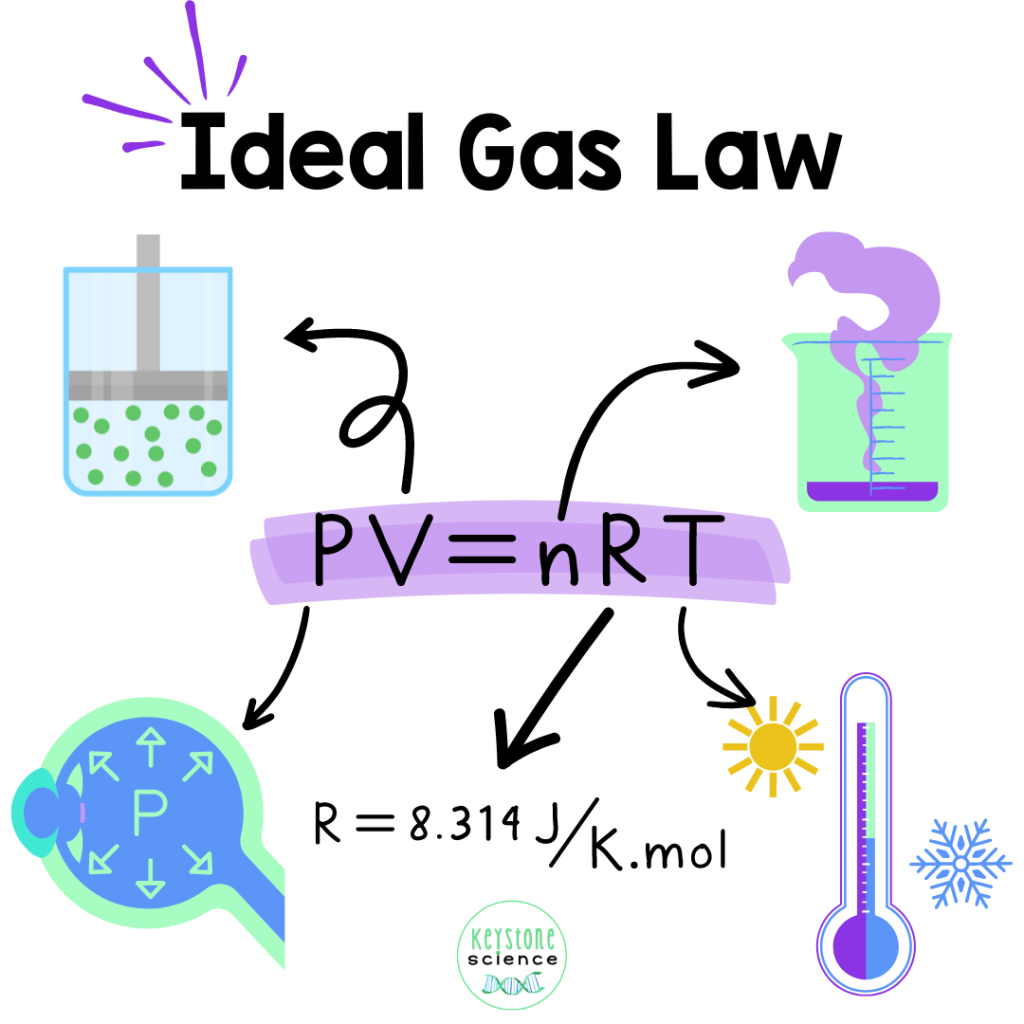
Once students grasp the basics of the Ideal Gas Law, there are several ways to incorporate it into your curriculum.
Want help with a unit about gas laws that practically teach themselves?
Grab this Gas Laws Unit Plan.
The first option is to examine how, when a person inhales, the molar amount of air in the lungs increases, and so does the volume of their lungs (lungs expand when you breathe in). On the other hand, when you exhale, the molar amount of air in the lungs decreases, and therefore the volume of the lungs also decreases.
This demonstrates that the pressure and temperature are kept constant in the lungs and the only change is the volume and amount (molar amount) of air particles.
Another option is to use online simulations as a tool to solve problems using the Ideal Gas Law formula. Reliable resources teachers can use in class include pHET, an online platform with free interactive simulations for math and science, the Concord Consortium, which is a STEM resource finder, as well as the University of Texas simulation, where students can change data for pressure, volume, and temperature to see different outcomes based on Ideal Gas Laws.
A final option is to cover every gas law with this ready-to-use Chemistry Gas Laws Packet with Massive Problem Set. This resource has everything you need to ensure students’ engagement and ultimate understanding and application of gas laws! It has important conversions for gas law problems, worksheets for every gas law, and helpful hints for teacher and student reference. It even includes a comprehensive answer key where all work is shown, information used is highlighted within the problem, and how to set up and solve each problem is included to save teachers much needed time!
Looking Ahead
When it comes to gas laws, I’m always looking ahead on the calendar to make the tie to Mole Day on October 23 which commemorates Avogadro’s Number 6.02 x 1023. Bringing current events and fun science-themed holidays into the classroom is an ideal way to bring a bit of fun and humor to your science classroom!
Beyond Mole Day and gas laws, the focus shifts to engaging hands-on strategies to get students interested and excited for the intricate organization and awesomeness that is the periodic table!
Let’s Connect!
💡Feel free to explore Keystone Science for ready-to-use high school chemistry, biology, ecology NGSS aligned notes and activities.
💡Subscribe to my email list for weekly tips direct to your inbox.
💡Follow me on Instagram for daily tips, motivation, and facts you can use in your classroom!
🌟Share in the comments or email me directly 👉🏻 [email protected]