Hi friend! First, I hope you’ve been taking some time for yourself during the summer break to unwind and do a few activities that you’ve been meaning to get to. If you’re reading this during the school year, great! This is going to be the perfect place to start in the previously mentioned Chemistry mini-series on Gas Laws that I hinted towards in the Gas Laws Unleashed blog.
In case you’re wondering if Gas Laws serve a place in the Next Generation Science Standards, the answer is 100% yes! In this post we’re going to continue exploring gas laws!
The Gas Laws Unleashed reading focused more on a general overview of each gas law, strategy to introduce them as well as ideas for gas laws classroom demonstrations.
So many teachers find that keeping students engaged when covering gas laws is a struggle, but it’s important to remember that understanding the behavior of gasses is the key to it all!
First in the Gas Laws mini-series, we will compare Boyle’s Law and Charles’s Law and discuss how these concepts can be effectively taught through engaging demonstrations, using real world connections.
Exploring Gas Laws Overview
Here is a quick overview of the gas laws we covered in the Gas Laws Unleashed blog

1️⃣Boyles Law: aka “Squishing Gases”
Boyle’s Law: Relationship between pressure and volume at constant temperature.
2️⃣Charles’s Law
Charles’s Law: Relationship between volume and temperature at constant pressure.
3️⃣Gay-Lussac’s Law: The relationship between pressure and temperature
Gay-Lussac’s Law: Direct relationship between pressure and temperature. As temperature increases the pressure will also increase.
4️⃣Combined Gas Law: The Triple Threat
Combined Gas Law: Relationship between pressure, volume and temperature.
5️⃣Avogadro’s Law
Avogadro’s Law: Direct relationship between moles and volume. As moles increase, so does volume when both temperature and pressure are fixed.
6️⃣Ideal Gas Law
R is a constant that depends on the units used for pressure, volume and temperature.
For example:
R = 0.0821 L⋅atm/(mol⋅K) when pressure is in atmospheres, volume in liters, and temperature in Kelvin.
R = 8.314J/(mol⋅K) when using SI units, with pressure in pascals, volume in cubic meters, and temperature in Kelvin
Now that we’ve done a quick refresher of each law, it is time to dive into really breaking down the explanation, relevance and application of both Boyle’s Law and Charles’s Law.
Boyle’s Law: “Squishing Gases”
Here’s the key to making these concepts stick comes to introducing Gas Laws: It’s all about application and demonstrations.
A favorite approach of mine is to actually start with a simple demonstration or video clip and use this as your conversation piece.
Don’t even mention the name of the specific gas law, instead use a visual representation to engage the class and slowly start to piece together the explanation of what was just witnessed!
Let’s revisit Boyle’s Law again. It states that there is an inverse relationship between pressure and volume. Simply put, as pressure increases, volume decreases and vice versa. P1V1 = P2V2.
Depending on your teaching approach, students’ learning styles and classroom equipment you have available, it could be best to introduce Boyle’s Law with 🌎real-world examples or 🏫classroom demonstrations.
🏫If a vacuum chamber is something you have access to, then the marshmallow experiment is a must to watch its effect when pressure is added and removed from the chamber.
🏫The Cartesian Diver classroom demonstration is a great way to get those wheels turning on how this syringe effect happens! When pressure is placed on the bottle, the air inside the syringe also compresses and expands causing the rise and fall of the object.
https://www.canva.com/design/DAFpvWSyLOo/SxeHNG5Wbm4OpoC5jiPL9Q/watch?embed
Cartesian Diver Phenomenon by Alyse Johnson-Chilla
🌎From here you can shift into real-life examples demonstrating Boyle’s Law by using The Concord Consortium STEM site.
Quick note that in order to access the resources, you can create a FREE account with them.
Once logged in, there are a handful of great examples to show your class visual representations of Boyle’s Law to help students gain a clear understanding of how changes in pressure, especially at different elevations, can influence gas volume.
Aside from providing visual support, The Gas Laws and Human Biology helps to make Boyle’s Law applicable by demonstrating how we see and experience this principle in our daily life!
Think potato chip bags being expanded during travel and ears popping during a flight.
These are tangible examples that students can grasp, creating more of those light bulb moments 💡
By presenting Boyle’s Law in a more exploratory approach, it helps to break down the concepts from just random variables to personal connections. Boyle’s Law is also a foundational piece in understanding how our atmosphere works and even meteorology- a perfect way to tie in Boyle’s Law and also hitting those Science and Engineering Practices and Crosscutting Concepts.
The Properties of Gases and Gas Laws ppt with guided student notes and the Free Boyle’s Law practice problems are 2 ready-to-use NGSS aligned content that walks you through ALL of the gas laws!
Do yourself a favor and snag these lessons so you can stop asking yourself “how am I supposed to…” and instead focus on which demonstrations will hook your students the most.
Charles’s Law: Temperature’s Impact on Gases
Charles’s Law explains the impact of temperature on gasses by demonstrating the relationship between volume and temperature at constant pressure. V1/T1 = V2/T2.
The illustration below shows a perfect explanation to what is observed when varying temperatures are applied to gasses and volume.
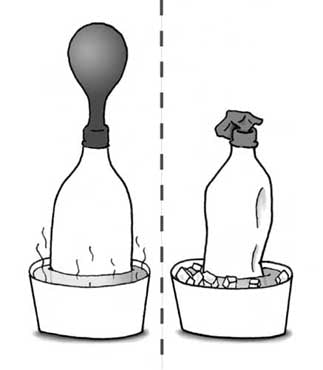
I’m sure you’re wondering, what are a few simple Charles’s Law classroom activities that can simplify this concept. Well, here you go 👇
🏫Classroom demonstration ideas:
🏫Charles’ Law Balloon Demo: All you need are balloons, 1 large beaker, hot / boiling water and a freezer.
Inflate 2 balloons to the same size and place each to a different temperature by adding one to the beaker holding hot water and the other in a freezer.
An alternate method is leaving one in the classroom and the other in a freezer.
Next, students will be able to observe volume changes with temperature changes.
🏫Collapsing Can: This is always a fun demo that will get a great reaction 😯
To set up, grab an empty soda can, a hot plate, heat resistant gloves, tongs and a large bowl of water.
Add a small amount of cool water to an empty soda can, apply heat to the can and after a few minutes, using gloves and tongs, quickly flip the heated can into the cool water and observe the rapid volume change!
Plus the “ooh’s and ahh’s” are always the cherry on top of this science demo 😎
🏫Tea light and Water: While not as exciting as the Collapsing Can demo, another approach to observe Charles’s Law is lighting a tea candle in a shallow pan of water. Place a small beaker over the candle and observe how water is “sucked” into the beaker as the air temperature cools following the candle going out.
https://www.canva.com/design/DAGGRG49JYQ/Zd7R-w9AqqYtGdnVSE1QCQ/watch?embed Charles Law Candle & Jaw Demo Video by Alyse Johnson-Chilla
🌎Real-life demonstration ideas:
Another resource from The Concord Consortium is the Gas Laws and Weather Balloon virtual activity.
Start by asking your students this question:
“Does anyone know how our weather is predicted?”
Once thoughts and ideas are shared, display an image of a weather balloon and see if this helps to connect more dots in explaining how a weather balloon works.
When applying Charles’s Law to weather, it explains the behavior of gasses in the atmosphere as the temperature warms and cools.
The Gas Laws and Weather Balloon activity supports those visual learners while allowing you to manipulate variables to see its effect of temperature on volume.
This virtual simulator along with the Properties of Gases and Gas Laws ppt and Charles’ law practice problems are excellent activities to emphasize those SEPs and CCCs that are essential in making concepts stick!
Comparing Boyle’s Law and Charles’s Law
Here is a comparison chart that highlights the key aspects of Boyle’s Law & Charles’ Law:
| Variables Involved | Mathematical Relationship | Constants & Conditions | |
| Boyles’ Law | Volume (V)Pressure (P) | P1xV1x = P2xV2 | Temperature (T) is constant |
| Charles’ Law | Volume (V)Temperature (T) | T1/V1 = T2/V2 | Pressure (P) is constant |
This chart can be used to illustrate the similarities and differences between the two gas laws, including the variables involved, their mathematical relationships, and the conditions under which they apply.
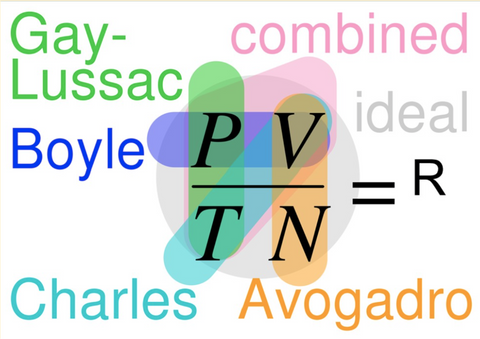
I’m guessing that if you were to present the image above to your students that there would be a look of confusion and the infamous “Do we need to memorize this?” question!
Fortunately, the Gas Laws mini-series does introduce and compare other concepts and the goal is that this Gas Laws summative illustration will provide much more clarity than it would if this images was shared on Day 1.
Integrating Demonstrations into Lessons
Before you click out of this page…if there is one piece of information you take away from this first gas laws mini-series, it’s that the key to fostering a scientist mindset is to always include demonstrations into your lessons.
Don’t worry about having the best or newest equipment! Sometimes, simple set-ups is all a classroom needs to spark ideas and conversations.
Afterall, science is all about experimentation, testing and observations. I know as teachers we can get hyper focused on state standards and testing dates, but what does it all matter if our lessons have no meaningful impact?

So here’s your goal before introducing your next gas law unit:
- Give a brief introduction into how gasses impact our day-to-day
- Pick a hands-on demonstration to engage your class
- Feel free to have students use the Phenomena Introduction Guide to brainstorm questions and explanations (P.S. it’s completely free!)
- Lead a group discussion on what was observed
- Finally, put all the pieces together explain what was just demonstrated
This Gas Laws Unit Bundle is a Keystone Science favorite!

❌No more spending hours searching online for individual worksheets.
✅The Gas Laws Unit bundle has a presentation filled with visuals, animations, 12 page gas laws practice problems, a student formula sheet and teacher answer keys too!
Conclusion
While I hope these classroom demos, simulations, presentations and practice worksheets are helpful, it’s important to consider how your students retain new content while always adding in your personal teaching style 🪄You know your students best.
As long as focus is kept on real-world examples, applications and engagement, then eventually comprehension will come. It might be at different points within a unit, but it will happen!
When you’re ready to start giving your gas laws curriculum a refresh or if you are looking to change things up a bit when it comes to unit labs and activities, then head over to visit Keystone Science where you can join the community and receive support via email, instagram and curriculum resources.
To be the first to know when each Gas Laws blog mini series is released this summer and other tips, I would love for you to join the Keystone Science Community!
If you’re wanting more, here you go:
💡Feel free to explore Keystone Science for ready-to-use high school biology & ecology NGSS aligned notes and activities.
💡Subscribe to my email list for weekly tips direct to your inbox.
💡Follow me on Instagram for daily tips, motivation, and facts you can use in your classroom!
🌟Share in the comments or email me directly 👉🏻 [email protected]