I’m curious what your take is on this topic 👇:
Every year, this is a debate amongst chemistry teachers whether or not Gas Laws belong in the Next Generation Science Standards. If they do, then where in the standards should it be taught?
If this is a topic that you’ve been unsure of or you’ve overheard your colleagues having the same debate, I’m here to tell you, YES, Gas Laws belong in the NGSS.
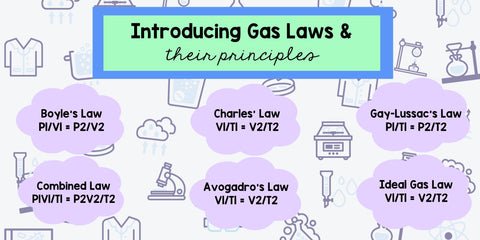
Gas laws align perfectly when teaching any of the Performance Expectations, cause and effect of the Cross-Cutting Concepts and Science and Engineering Practices can be applied, depending on the design of your lesson and learning goals.
Let me break down the standards that align, how the Gas Laws apply and the PE connections to NGSS Physical Science:
- HS-PS1-3 Matter and its Interactions
- HS-PS2-4 Motion and Stability: Forces and Interactions
- HS-PS3-2 Energy
- HS-PS3-4 Energy
- HS-PS3-5 Energy
- HS-PS1 Matter and its Interactions
- HS-PS2 Motion and Stability: Forces and Interactions
- HS-PS3 Energy
You might have noticed that the Gas Laws unit is a bit more complex in students understanding the how behind the laws.
Truly, the key to understanding the Gas Laws lies in the method that you are presenting them!
If you’re stuck in finding an effective method or you are in your first year of teaching and you want to be better prepared, then you’ll want to keep checking in 🙌
This will be the first in a mini series of Gas Laws articles where I will dive deeper into what each law is, phenomena of gas laws, modeling and suggested graphic organizers and activities for each principle. So stay tuned for more support on this topic!
Here I will provide you with more of a guideline into how to introduce Gas Laws, strategies to engage your students and some real-world applications.
The question that always comes up is…
➡️ “What is the best way to teach gas laws?”
In education, you know that there is never a one-size fits all. Your approach to teaching will always depend on your students, their learning style, your expertise and most of the time what equipment your school has available to you.
Regardless of these factors, the most important piece that you will always want to focus on is combining Gas Laws to real life examples.
For example, just defining Boyle’s Law and showing its formula means absolutely nothing without offering relatable context. We’ve covered in earlier posts just how powerful real-world application has on the learning process and the value it brings to students finding connection to the lesson.
Think about this blog post! What was it that made you decide to click, read and continue reading? It was the fact that you have a connection to this topic! Maybe you thought “I need this because Gas Laws is the next unit I’m covering” or “I struggle with how to make Gas Laws relatable”
Whatever the thought, you had a connection to the topic, it kept you engaged and curious for more.
The same goes for our students. When you are able to show how a topic relates to their everyday life, there’s a small light that turns on and now you have their attention.
➡️Here is a go-to practice activity on Pressure, Temperature and Mole Conversions with the answer key, perfect for converting between Celsius and Kelvin, units of pressure (psi, kPa, mmHg, atm), and between moles and liters.

Before we head into classroom demonstrations for Gas Laws, it’s important to note that this unit also aligns with CCC’s and SEP’s.
The CCC’s that tie into this particular unit include:
- Cause and Effect
- Scale, Proportion and Quantity
Cause and Effect includes using empirical evidence that is required to differentiate between cause and correlation and finally making a claim about the specific causes and effects.
Scale, Proportion, and Quantity uses algebraic thinking to help break down scientific data and predict the effect of a change in one variable on another.
SEPs highlight students utilizing practices to mirror those of true scientists and engineers. What better way than to create models to help support a claim!
The Wonder of Science explains that models don’t necessarily need to be physical models. Instead these can include diagrams, mathematical formulas or analogies to help represent a system or parts of a system.
A benefit to models is the practice in developing questions, explanations and making predictions. Watch out for future posts about how to use modeling with each Gas Law!
Sequence to Teaching Gas Laws and Classroom Demonstrations
At this point, we’ve covered:
- Where Gas Laws align with NGSS Physical Science standards
- The importance of real-world applications
- How modeling can support the predictions and explanations
Let’s get into the sequence of introducing Gas Laws and classroom demonstrations to use with them.
🧑🏫Demonstrations as a Gateway
One of the most simplistic strategies to help with student engagement is by using classroom demonstrations to help break down a complex concept with a simple model.
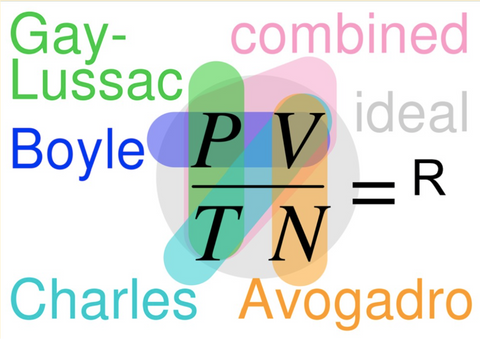
Imagine displaying this Gas Laws image and expecting your classes to understand the meaning. If your students are like mine, you would see a lot of confused expressions or probably hear “Are we going to have to memorize that?!”
By setting up easy demonstrations, it will create natural curiosity and right away you have an engaged class.
Plus, the magic in demos is that you can introduce Gas Laws without even mentioning the actual principal, students are in the driver’s seat of their own learning and you can tie in 3 Dimensional Learning, all with this ONE approach!.
1️⃣Boyle’s Law: “Squishing Gases”
- What is Boyle’s Law?
Boyle’s Law states that there is an inverse relationship between pressure and volume. Simply put, as pressure increases, volume decreases and vice versa.
The formula demonstrates the inverse relationship between the two variables: P1V1 = P2V2
But remember, the focus is engagement and connection. So before going straight into the Properties of Gases & Gas Laws lecture notes, introduce Boyle’s Law through a demonstration.
💥Boyle’s Law Demonstration💥
Use the Cartesian Diver to naturally lead into Boyle’s Law.
This setup could either be done with you demonstrating the law in action or depending on the resources available, you can also set up the Cartesian Diver demo within small groups and while they interact with the model, ask questions to lead them to an explanation of what might be happening.
Cartesian diver explanation: When the sides of the bottle are squeezed, the pressure on the water increases, causing the air inside the diver to compress. This decrease in volume of the air increases the overall density of the diver, making it denser than the surrounding water and causing it to sink to the bottom of the bottle. Conversely, when the pressure is released, the air inside the diver expands, decreasing its density and causing it to float back to the surface
Continue to create conversations by asking questions like:
“What do you think this means?”
“Give an example of how we interact with pressure in our environment?”
“What does volume mean?”
“How might the demonstration relate to the human body in the lungs?”
Another favorite engagement strategy is to show virtual demonstrations. The Concord Consortium offers a great interactive tool, Gas Laws & Human Biology which shows different real-world applications of Boyle’s Law.
Once you see that your classes have a better understanding of how we interact with Boyle’s Law, start to piece together the more scientific and mathematical elements.
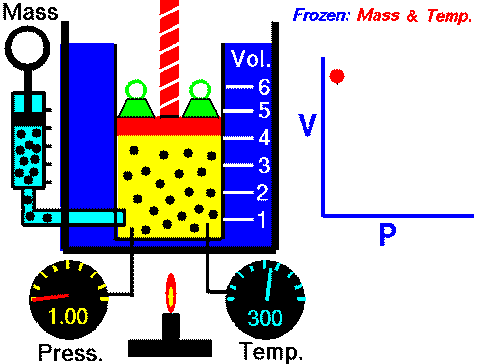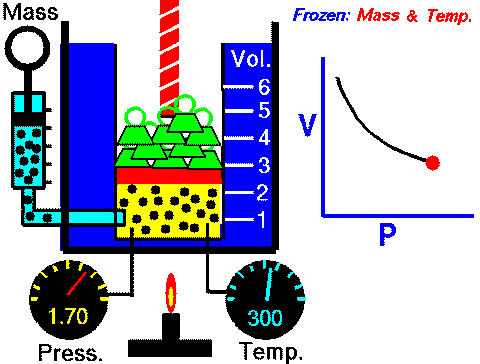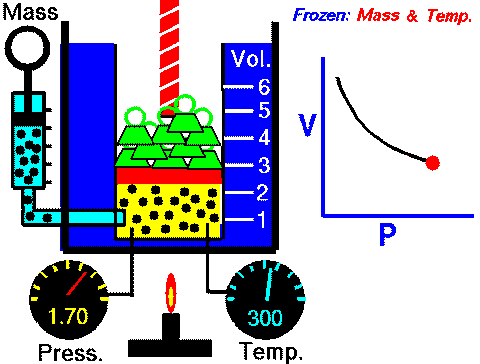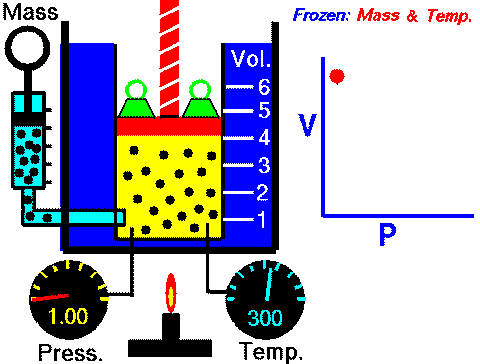
In case you are wondering, this diagram can be found in the Properties of Gases and Gas Laws ppt with guided student notes where students can better visualize the variables and how they change in each law.
2️⃣Temperature’s Impact on Gases
- What is Charles’ Law?
Charles’ Law states that there is a direct relationship between volume and temperature.
The formula here demonstrates this direction relationship: V1/T1 = V2/T2
But, before sharing its definition or formula, we want to focus on keeping our class engaged.
💥Charles’ Law Demonstration💥
Introduce Charles’ Law with a balloon demonstration to show inflation at different temperatures.
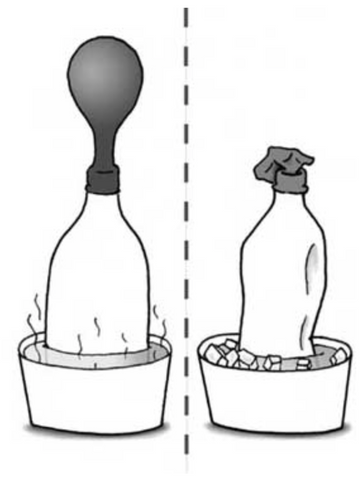
Allow time for questions, conversations and reminding your students to build upon what they learned in Boyle’s Law.
- “What variable is being added in this demonstration?”
- “How does this variable affect the gas in the balloon?”
For a virtual demonstration, try the Concord Consortium Gas Laws & Weather Balloons interactive tool.
By carving out time for showcasing either physical or virtual models, you are also helping to build confidence when leading into more complex principles.
3️⃣Pressure and Temperature Relationship
- What is Gay-Lussac’s Law?
Gay-Lussac’s Law explains that there is a direct relationship between pressure and temperature, stating that as temperature increases the pressure will also increase.
P1/T1 = P2/T2.
Considering that real-world application is key, try either setting up a pressure cooker if you have one or displaying how a pressure works through video.
💥Gay-Lussac’s Law Demonstration💥
FIRST: Breakdown how a pressure cooker works while identifying parts of the equipment:
If you have a pressure cooker that you can use, excellent, if not, a picture will do just fine! Identify and explain the function of each part: Material, sealed container, liquid added, sealed lid.
SECOND: How does a pressure cooker simulate Gay-Lussac’s Law?
Ask your class and see if they have seen the pressure being used! If so, what did they observe while it was heating, how was it opened, etc.
Be sure to give a general explanation of its functions as far as: heating, increased pressure, fast cooking and releasing heat.
THIRD: Connecting back to Gay-Lussac’s Law
Now that the parts and functions of a pressure cooker have been identified, provide leading questions to see if your classes can help explain how a pressure cooker uses Gay-Lussac’s Law.
The biggest takeaway that students need to understand is that by regulating the heat, the pressure inside the pressure cooker can be controlled.
4️⃣Triple Threat: The Combined Gas Law
- What is the Combined Gas Law?
The Combined Gas Law, by describing the mathematical relationship between pressure, volume and temperature. P1V1/T1 = P2V2/T2
Remember that super complex diagram summarizing the 3 variables of gas? Yes, this one 👇
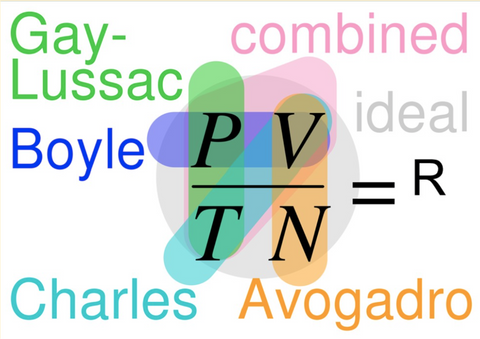
Before using this to explain the Combined Gas Law and hearing “ARE WE GOING TO HAVE TO MEMORIZE THAT?!” Here is another classic crowd pleaser you can use instead.
💥Combined Gas Law Demonstration💥
The Crushing Can is always a fun one!
Essentially, the heated can with water will crush when placed upside down in cool water. Be prepared to have multiple empty cans to repeat this because your students will love it 👏
If virtual demonstrations or getting your students into small groups is something you like to incorporate, try using The Concord Consortium: Crushing Railcar Phenomena
5️⃣The Return of the Mole: Avogadro’s Law
- What is Avogadro’s Law?
Avogadro’s Law states that there is a direct relationship between moles and volume. As moles increase, so does volume when both temperature and pressure are fixed. V1/N1=V2/N2
There is a new variable that is being introduced. Moles, a unit of measurement for large quantities of smaller substances such as atoms, molecules and other particles.
Without going into too much about the mathematical calculations, introduce Avogadro’s Law through this easy demonstration.
💥Avogadro’s Law Demonstration💥
Using empty bottles, baking soda and vinegar, add varying amounts across each bottle and see if your students can guess which bottle will have the least amount of gas produced in the balloon.

Finally having covered Boyle’s, Charles’, Gay-Lussac’s and Avogadro’s Laws, this is a perfect transition into the Ideal Gas Law.
6️⃣Bringing It All Together
Now that each law has been covered, the Gas Laws overview image might make a bit more sense when displayed…
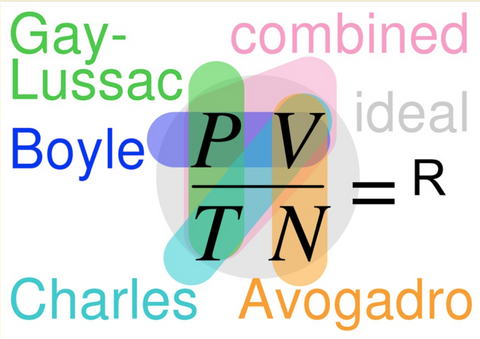
Consider using the diagram to ask brief review questions, which could be used as an informal assessment, of their level of understanding.
If you need to go back to one or two of these laws, take the time to do that before introducing R, the newest variable.
- What is the Ideal Gas Law?
R is a constant that depends on the units used for pressure, volume and temperature.
For example:
R = 0.0821 L⋅atm/(mol⋅K) when pressure is in atmospheres, volume in liters, and temperature in Kelvin.
R = 8.314J/(mol⋅K) when using SI units, with pressure in pascals, volume in cubic meters, and temperature in Kelvin.
This is where you might see some of those confused faces, so it’s important to use multiple examples and virtual illustrations of how these laws work together.
💥Ideal Gas Law Demonstration💥
Depending on the learning style and needs of your students, you can choose to practice these laws with worksheets or through technology. For general review, the Gas Laws Practice Packet offers a nice overview of each principal along with practice in conversions.
If technology is the route you prefer to review, then these are incredible tools you might want to consider:
- An excellent summary of the Gas Laws can be found on the Concord Consortium: Gas Laws simulation.
- If you are looking for more of an all-in-one Gas Laws simulator, try the University of Texas Ideal Gas Law simulator.
- Finally, once students have a better concept of how each of the variables work both independently and with each other, PHET Interactive Simulations has a great site where students can manipulate each variable on its own!
Conclusion
Remember, going into Gas Laws is just one of those units where you will want to provide as many hands-on activities and demonstrations as possible alongside real-life applications.
If you are limited in supplies or equipment, don’t worry! Just make sure to incorporate virtual interactive simulators, videos, practice problems and have a thorough Gas Laws presentation with student guided notes filled with diagrams easy to interpret.
Before introducing the unit, take a look at your current curriculum and see where you can add in more visuals and practice.
If this is your first year teaching Gas Laws or you are just wanting to start with fresh content, you can grab the Gas Laws Unit Bundle, where it has everything you need from:
- Interactive PowerPoint presentations
- Student-led notes
- Gas Laws formula sheet
- Gas Laws practice problems
- Teacher answer key with explanations to Gas Laws practice problems

I hope you feel better prepared to lead your students into this interactive unit and that the demonstrations were helpful in sparking ideas for creating engagement in your future lessons!
💡For more information on NGSS, visit https://www.nextgenscience.org
💡As a quick refresher on 3D learning and steps on how to apply NGSS, visit the Keystone Science Blog at https://keystonesciencepa.com/blogs/teachertips
💡Feel free to explore Keystone Science for ready-to-use NGSS aligned notes and activities.
💡Subscribe to my email list for weekly tips direct to your inbox.
💡Follow me on Instagram for daily tips, motivation, and facts you can use in your classroom!
I would love to hear your feedback, questions and success stories on this format of introducing gas laws and their principles !
Share in the comments or email me directly 👉🏻 [email protected]













